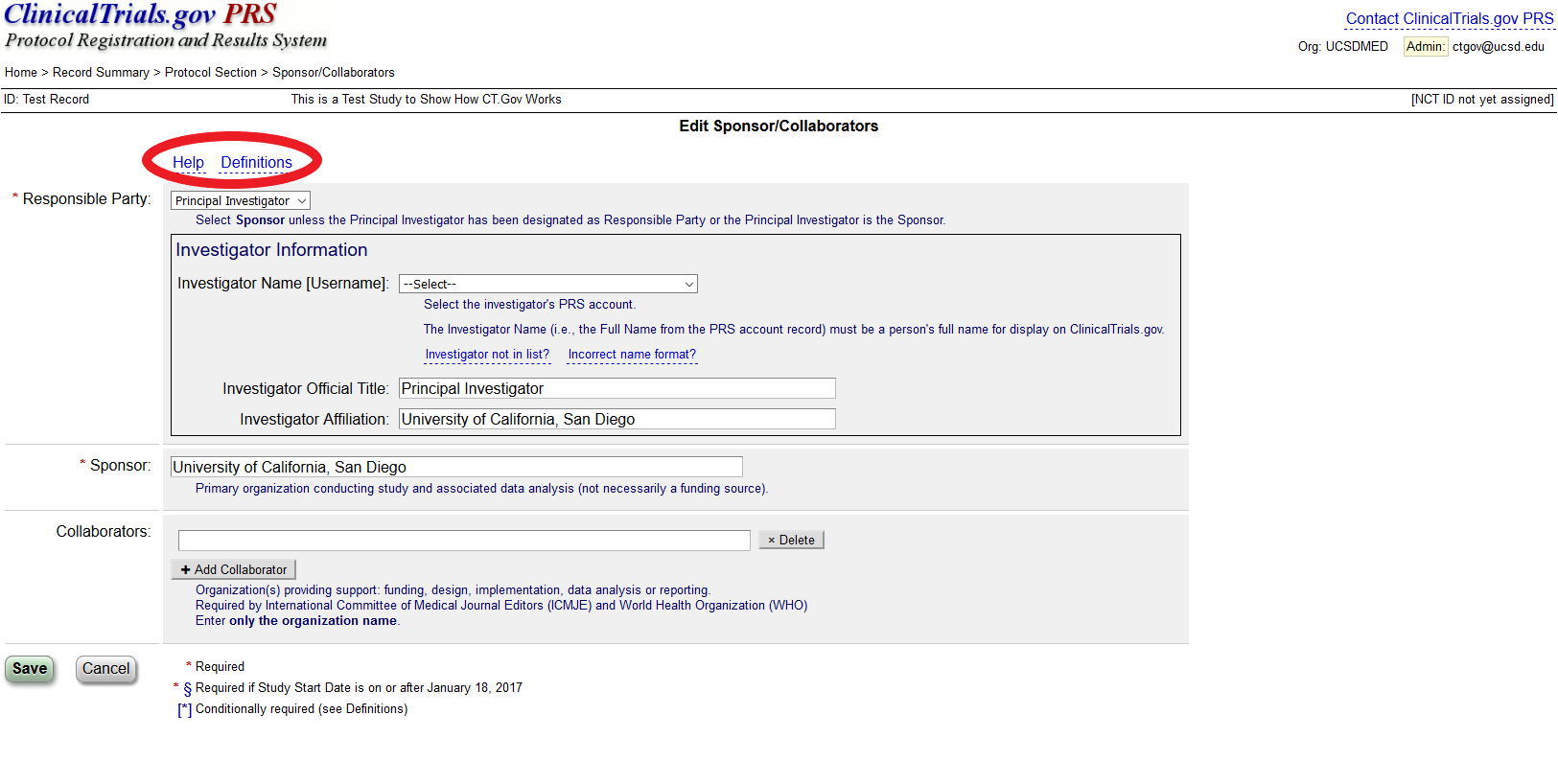
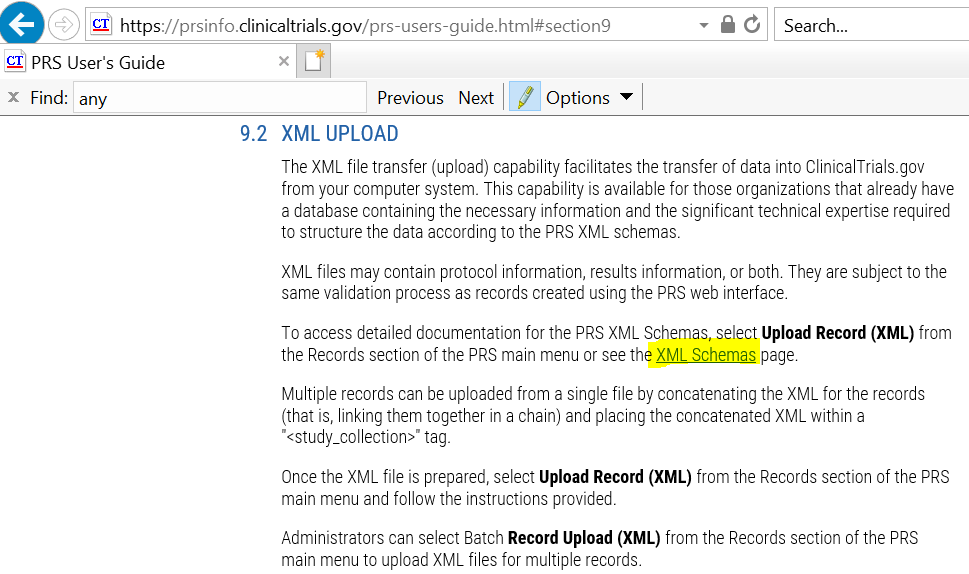
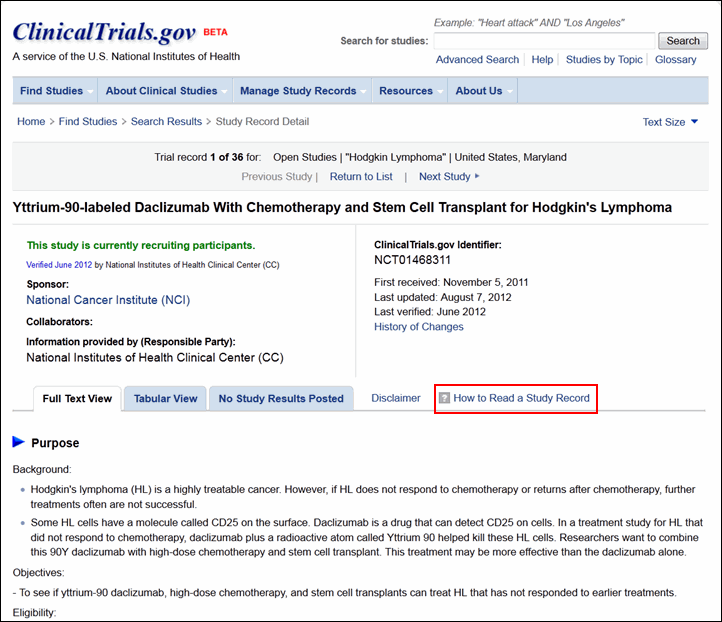
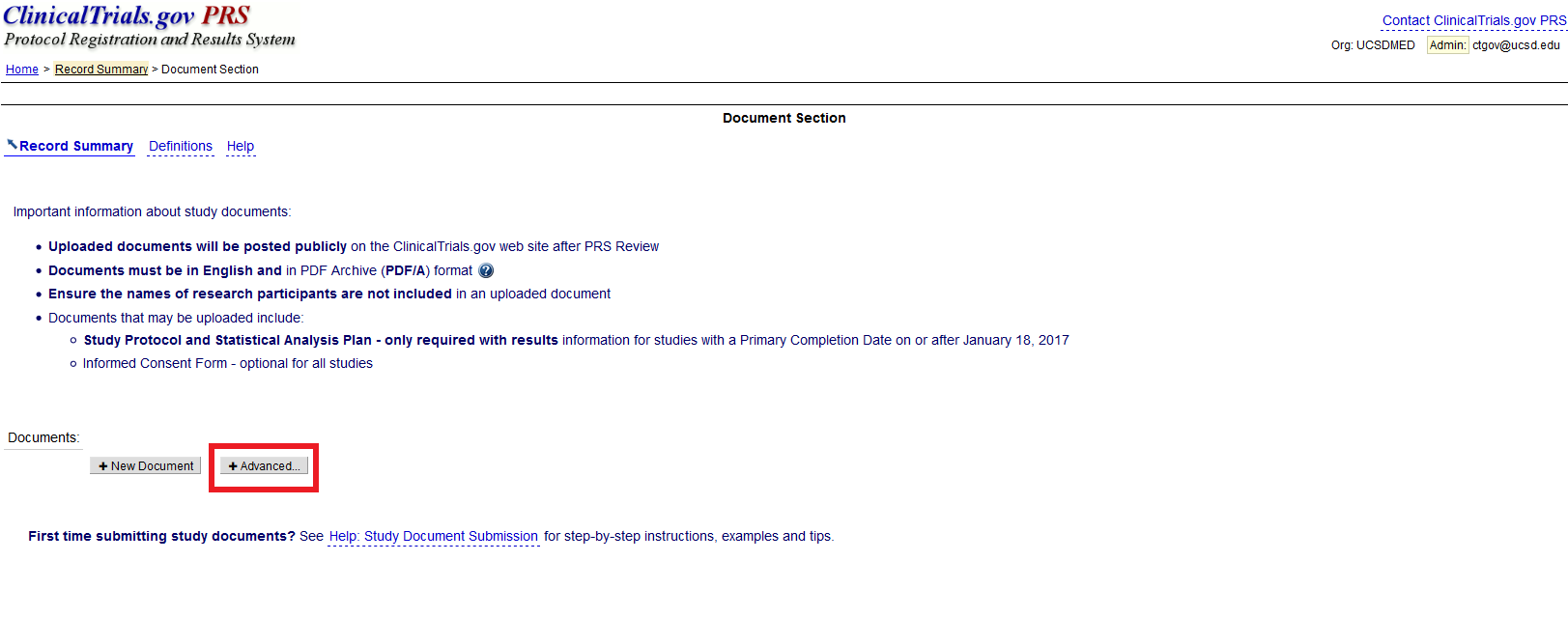
PDF) 3381 Reducing Problem Records in the Johns Hopkins University ClinicalTrials.gov Protocol Registration and Results System (PRS)
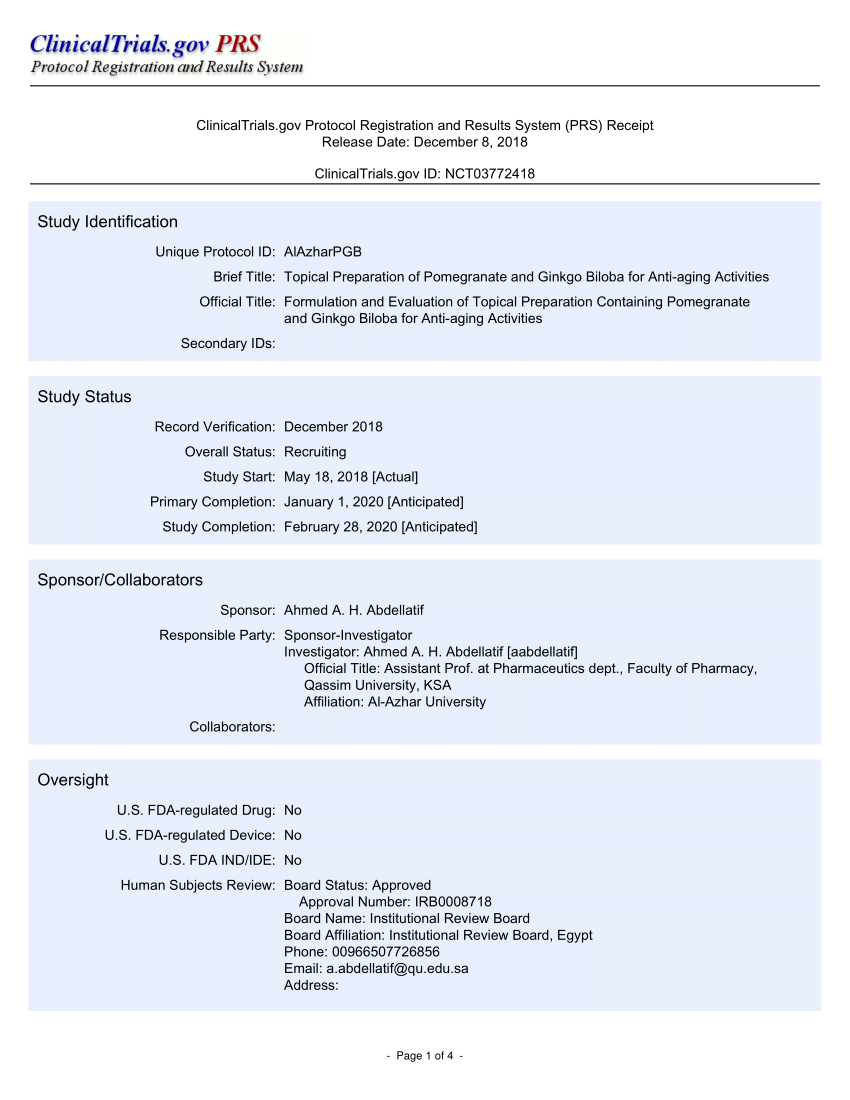
PDF) ClinicalTrials.gov Protocol Registration and Results System (PRS) Receipt Study Identification Unique Protocol ID: AlAzharPGB Brief Title: Topical Preparation of Pomegranate and Ginkgo Biloba for Anti-aging Activities Official Title: Formulation ...
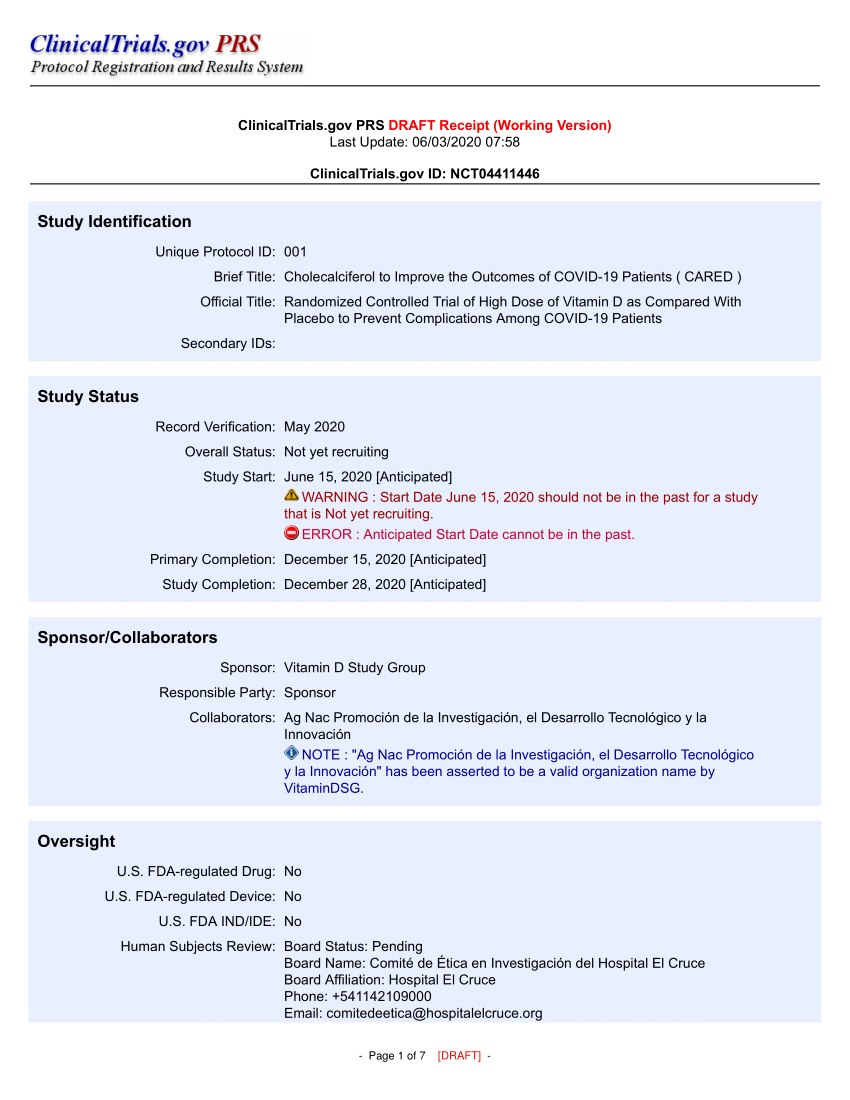
PDF) ClinicalTrials.gov Brief Title: Cholecalciferol to Improve the Outcomes of COVID-19 Patients ( CARED ) Official Title: Randomized Controlled Trial of High Dose of Vitamin D as Compared With Placebo to Prevent