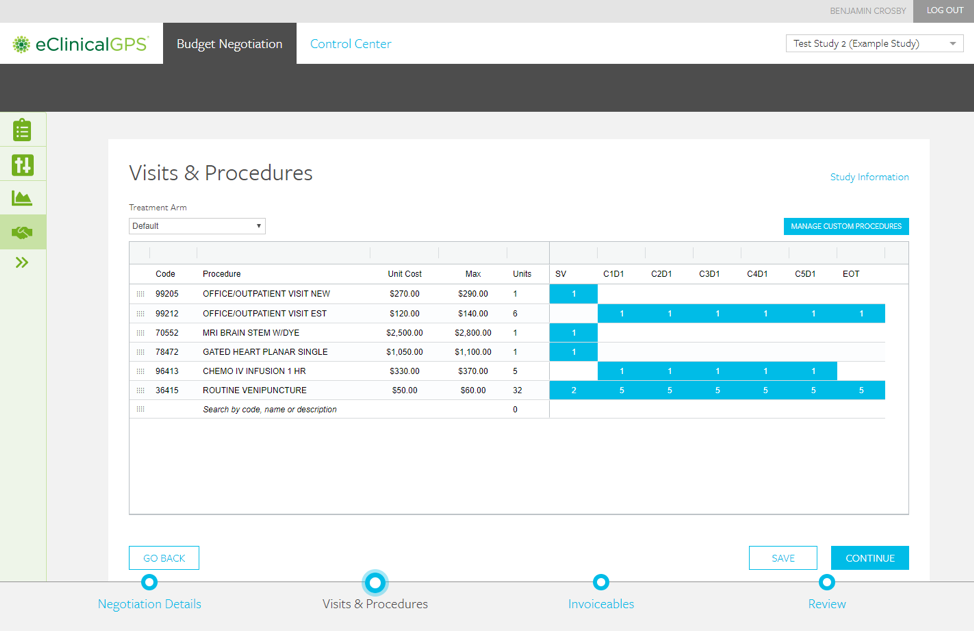
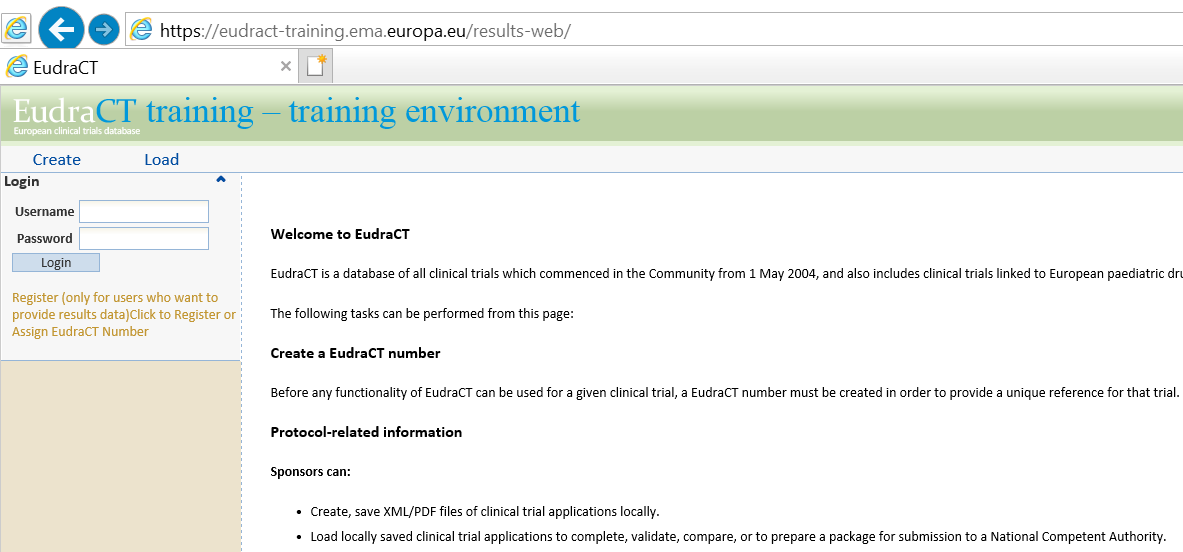
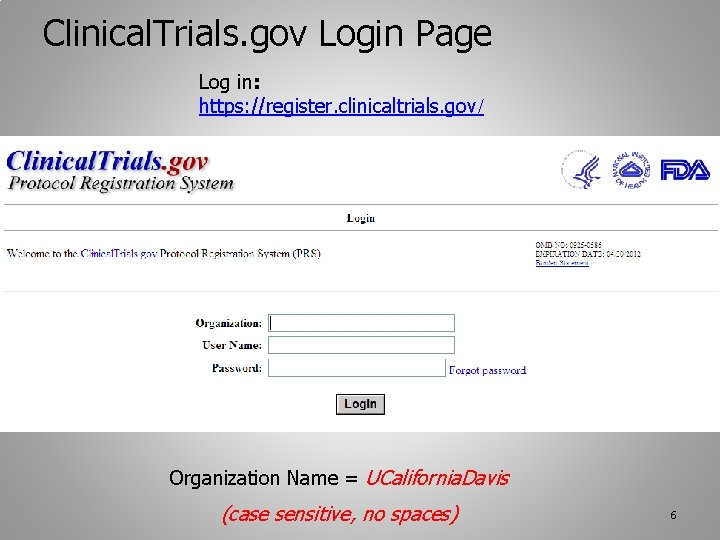
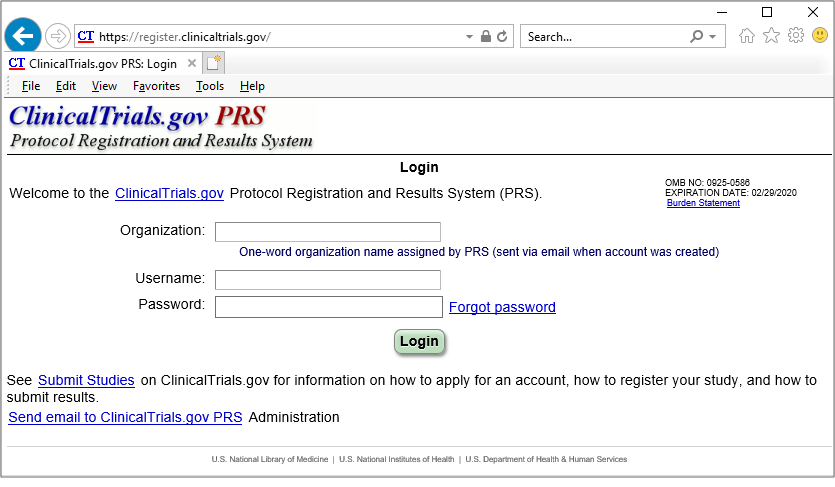
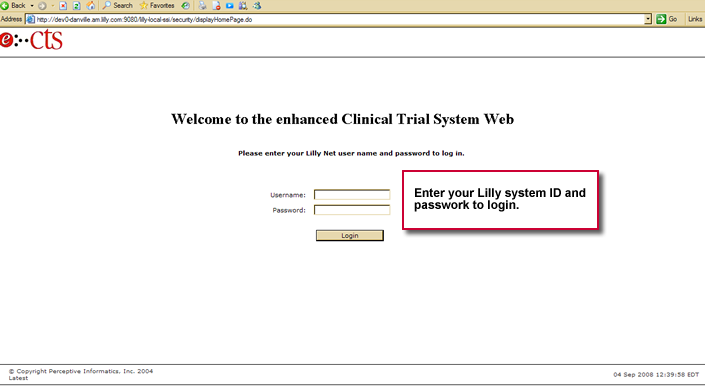
myTomorrows Now Offers HCP Portal with Integrations for Clinical Trial Referral and Team Collaboration

Process of registering a study in Clinical Trials Registry -India (a)... | Download Scientific Diagram

Chinese Clinical Trial Register (ChiCTR) - The world health organization international clinical trials registered organization registered platform

POS-426 CLINICAL TRIAL DATABASE (CTD): INTEGRATED DATABASE MANAGEMENT SYSTEM FOR CLINICAL TRIALS - Kidney International Reports

Ongoing Clinical Trials for the Management of the COVID-19 Pandemic: Trends in Pharmacological Sciences