Amid The Turmoil Of 2020, This Company Was The First To Treat Patients With A Genome Editing Therapy
JPM 2022: Editas, which caught flak in 2021 for limited gene editing data, will try to layer on the proof in 2022 | Fierce Biotech

Delivery of CRISPR-Cas tools for in vivo genome editing therapy: Trends and challenges - ScienceDirect

Leber congenital amaurosis/early-onset severe retinal dystrophy: current management and clinical trials | British Journal of Ophthalmology

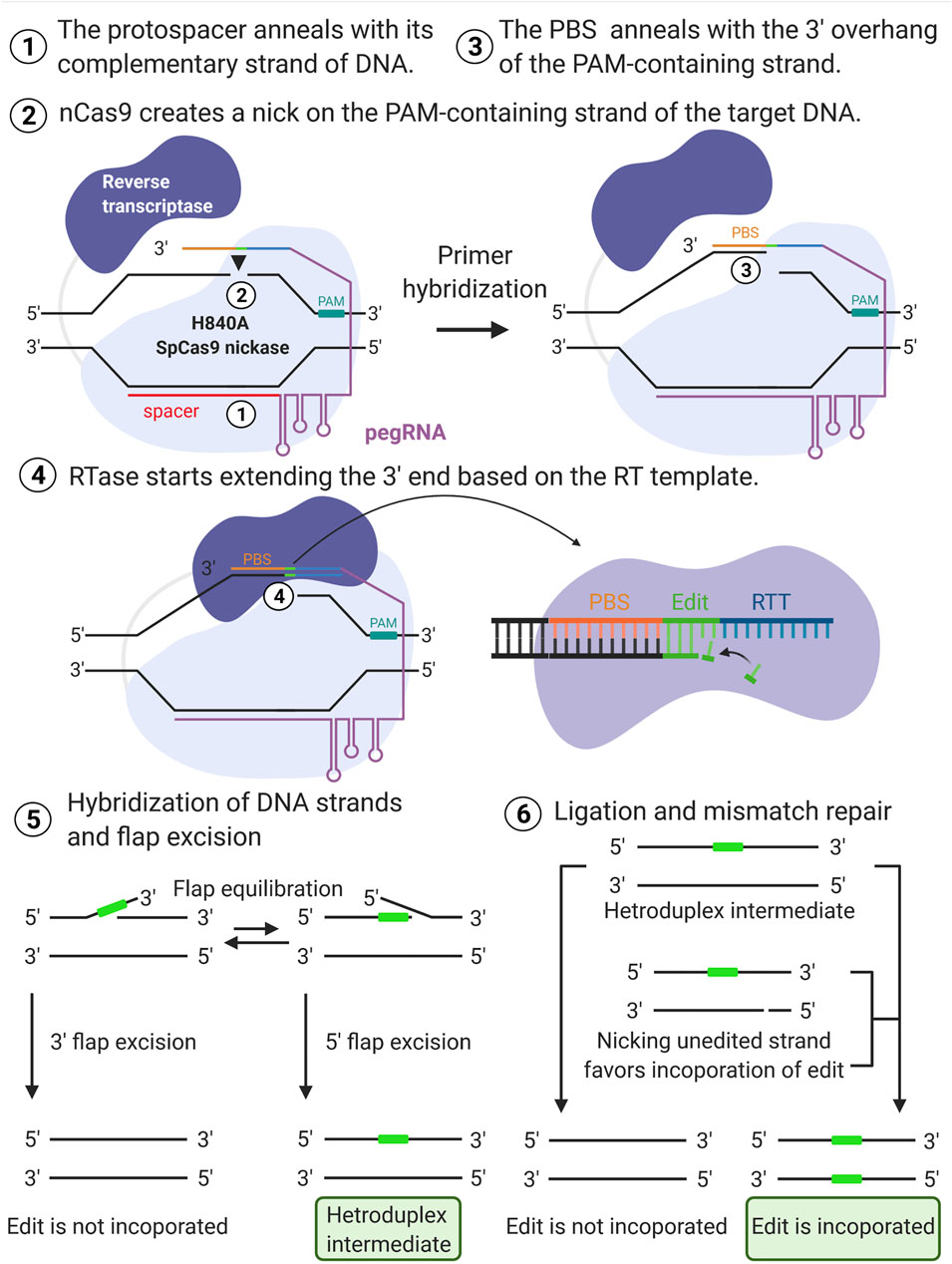
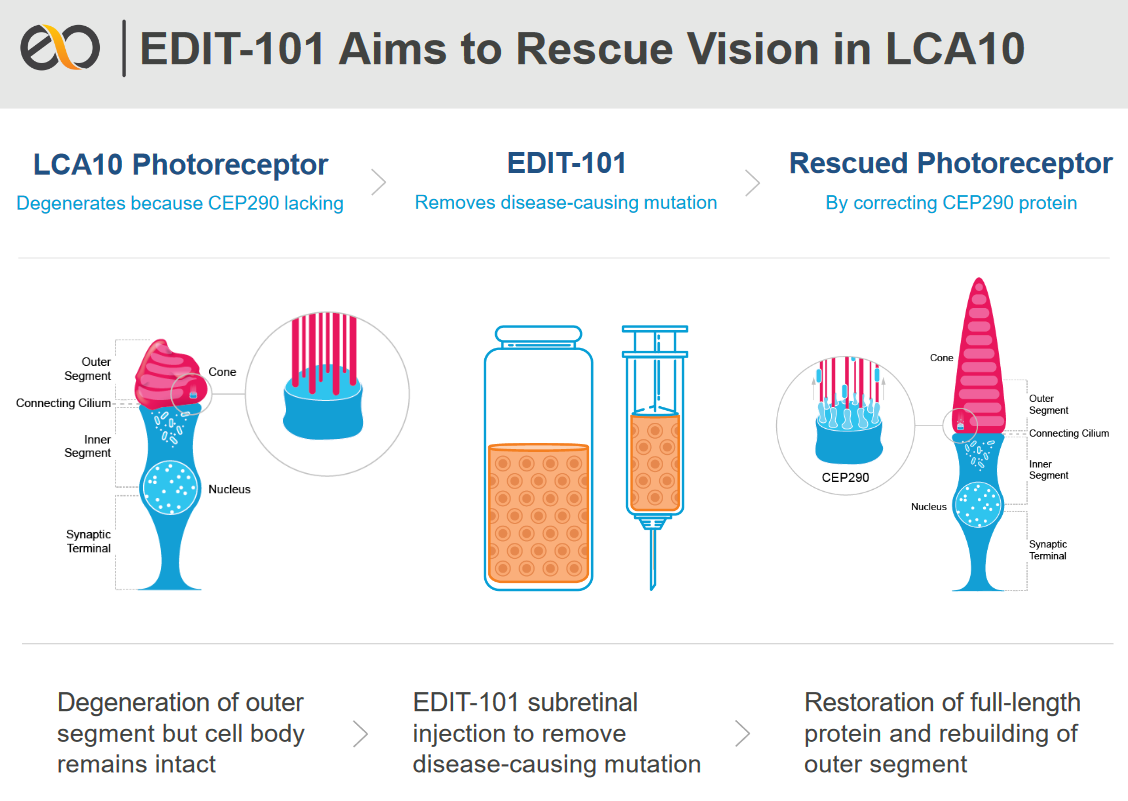
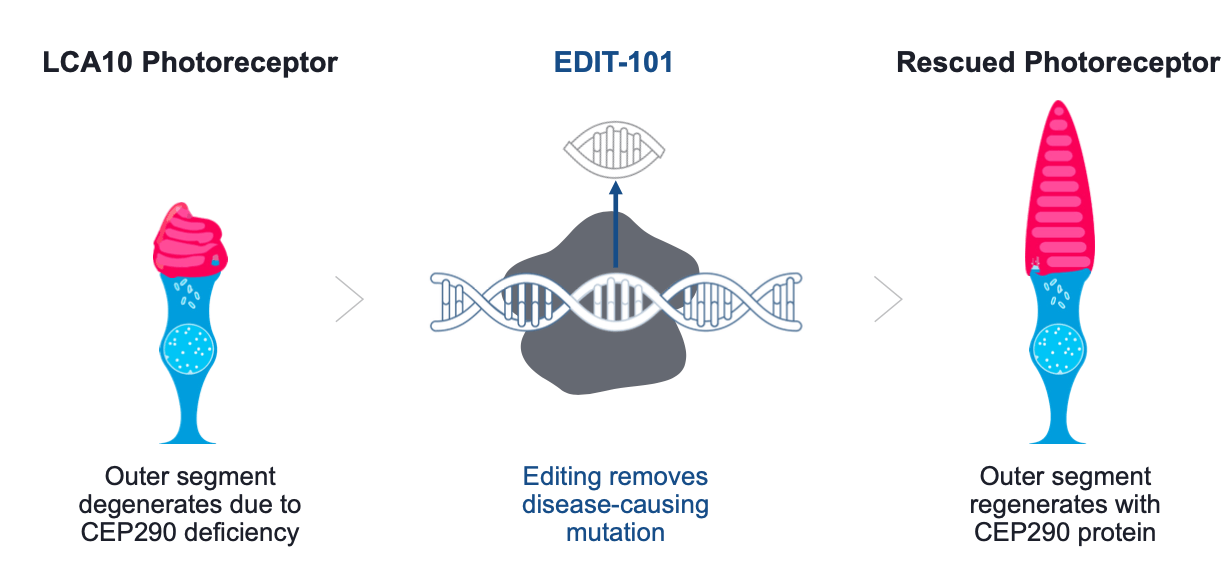
Development of a gene-editing approach to restore vision loss in Leber congenital amaurosis type 10 | Nature Medicine

Editas and Allergan Make Gene-Editing History With First Treatment of Blindness Drug | The Motley Fool

Yuancheng (Ryan) Lu on Twitter: "Editas has their gene editing AAV therapy application approved by FDA. Will start clinical trials. https://t.co/gD60RtSM6E" / Twitter

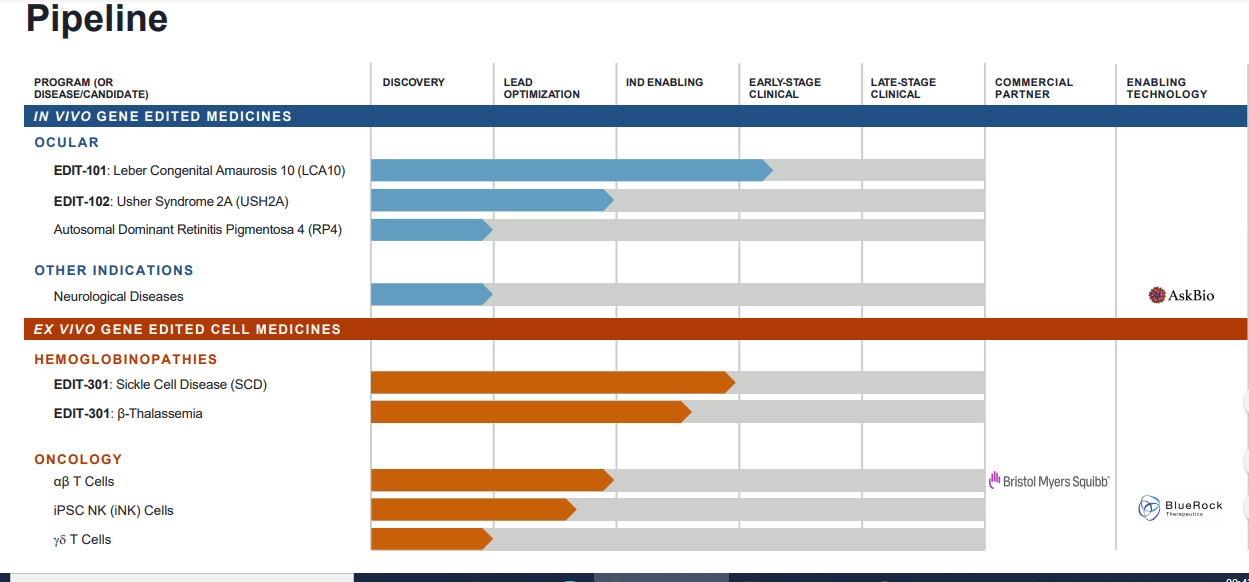
Editas Medicine Announces Dosing of First Pediatric Patient in the BRILLIANCE Clinical Trial of EDIT-101 for LCA10 | Editas Medicine
Editas Medicine stock slumps 15%, chief scientific officer to retire, provides trial updates (NASDAQ:EDIT) | Seeking Alpha