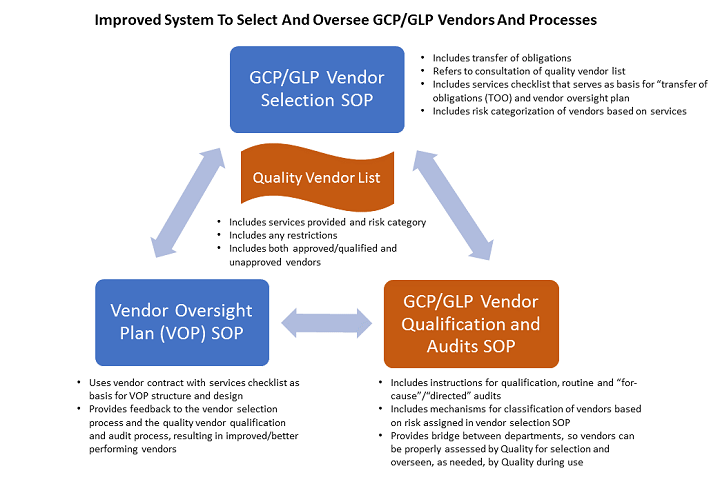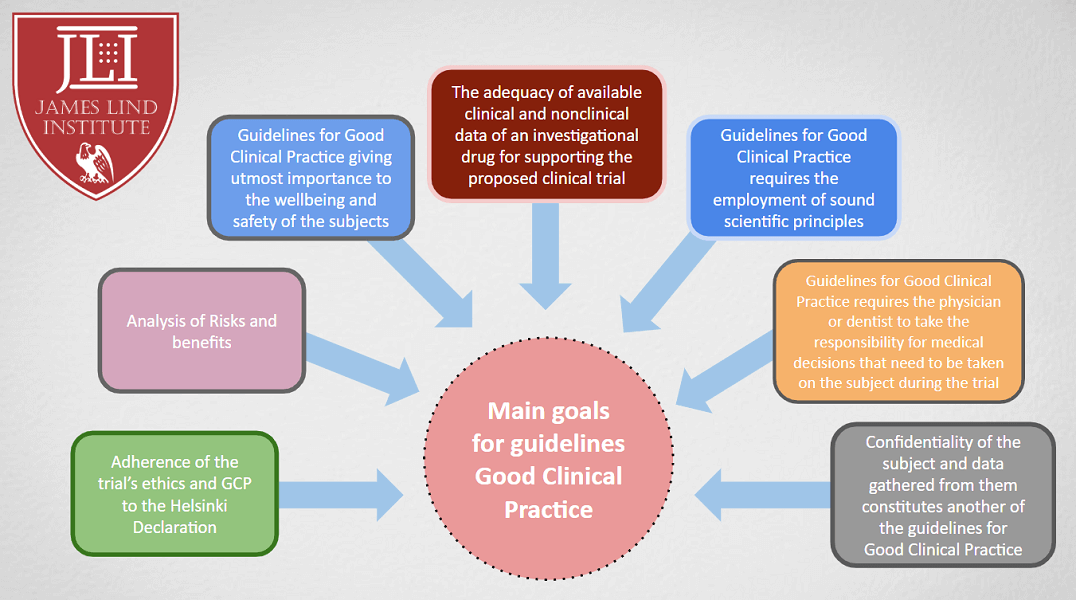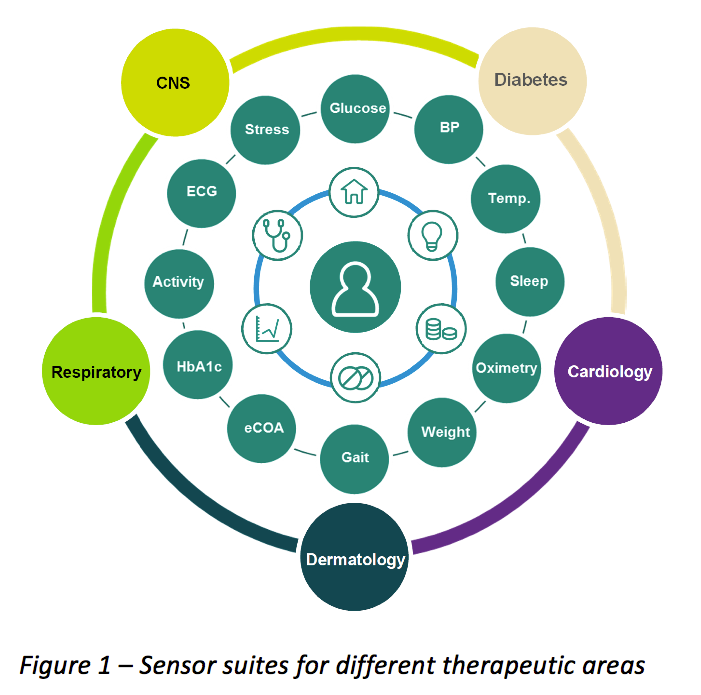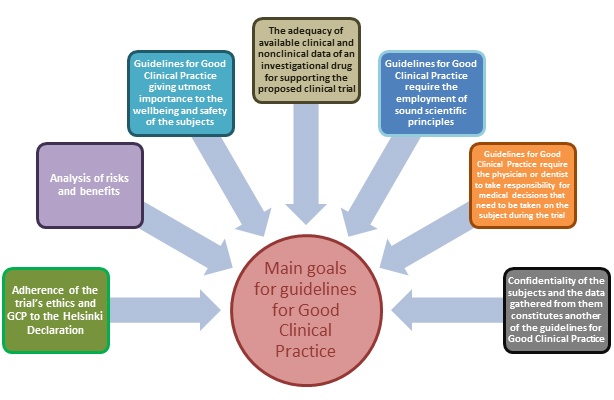
The Good Clinical Practice (GCP) and the responsibilities of pharma sponsors - Avantyo article in Viata Medicala magazine · News · Avantyo

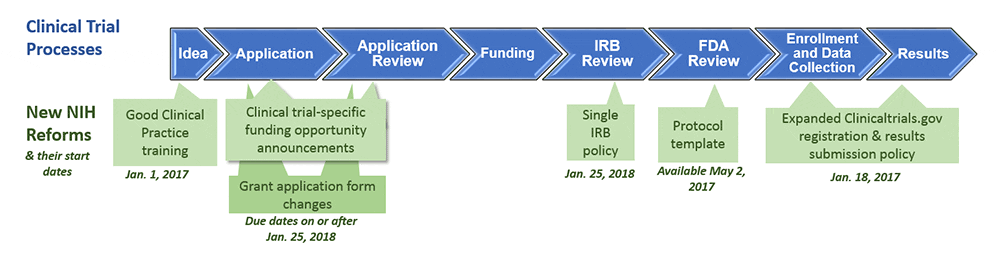
Good Clinical Practice (GCP) and Multi-Regional Clinical Trials (MRCT) Training - The Multi-Regional Clinical Trials Center of Harvard and Brigham and Women's Hospital

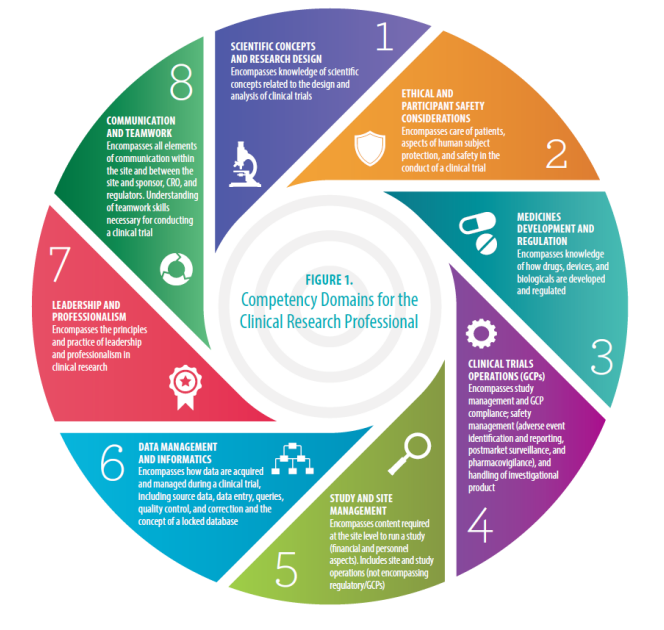
ICH Good Clinical Practice (GCP) E6 (R2) and regulatory requirements for Clinical Trials (Curtin University) - RETProgram

A GCP Primer: Understanding the Basic Application of Good Clinical Practice in the Regulated Environment for the Quality Professional | IVT - GCP

The Good Clinical Practice (GCP) and the responsibilities of pharma sponsors - Avantyo article in Viata Medicala magazine · News · Avantyo


(135).jpg)