ADVANCED CLINICAL TRIALS THE CLINICAL TRIAL PROCESS: IMPENDING CHANGES IN THE REGULATORY FRAMEWORK - ADVANCED CLINICAL TRIALS

CLINICAL TRIALS - Spotlight on Quality in Study Startup: Automated Workflows Encourage Upfront Planning & Downstream Improvements in the eTMF
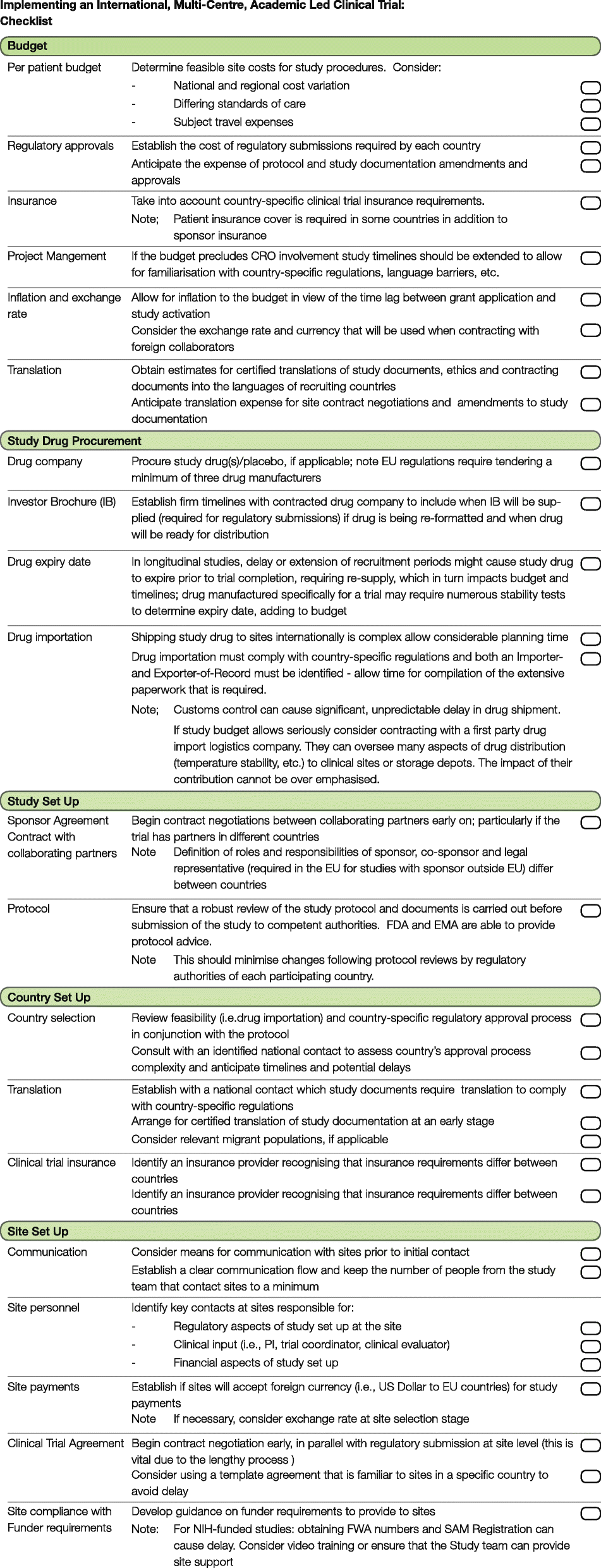
A checklist for clinical trials in rare disease: obstacles and anticipatory actions—lessons learned from the FOR-DMD trial | Trials | Full Text

Guidance Document: Part C, Division 5 of the Food and Drug Regulations “Drugs for Clinical Trials Involving Human Subjects” (GUI-0100) - Canada.ca

REGULATORY “ESSENTIAL” DOCUMENTATION Role of the RESEARCH COORDINATOR Best Practices 21CFR Part 11 Monday, November 7, ppt download